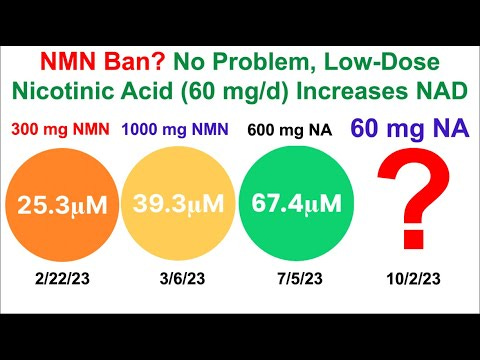
The loss of NAD in various disease states and why it is important, niacin flush is considered a healthy response, Nicotinic Acid may help restore NAD levels
https://pmc.ncbi.nlm.nih.gov/articles/PMC8082456/
The Key Role of NAD+ in Anti-Tumor Immune Response: An Update
Fabio Morandi 1,*, Alberto Leonardo Horenstein 2, Fabio Malavasi 2
Author information
Article notes
Copyright and License information
PMCID: PMC8082456 PMID: 33936090
Abstract
Nicotinamide adenine dinucleotide (NAD+) is an important molecule that functions as a co-enzyme in numerous metabolic processes. Generated both through de novo synthesis and via salvage pathways, NAD+ is the substrate for a variety of NAD+-consuming enzymes.
Among them is CD38, a cell surface ecto-enzyme widely expressed on different types of cells and endowed with the function of cADP-ribose synthases/NAD+ glycohydrolase.
Surface CD38 expression is increased in different hematological and solid tumors, where it cooperates with other ecto-enzymes to produce the immunosuppressive molecule adenosine (ADO).
Few studies have explored the correlation of NAD+ levels with T-cell mediated anti-tumor response in preclinical models. We therefore discuss these novel findings, examining the possible contribution of NAD+ depletion, along with ADO production, in the immunosuppressive activities of CD38 in the context of human tumors.
Lastly, we discuss the use of pharmacological inhibitors of CD38 and supplementation of different NAD+ precursors to increase NAD+ levels and to boost T cell responses. Such molecules may be employed as adjuvant therapies, in combination with standard treatments, for cancer patients.
Keywords: NAD+, human tumors, immune response, CD38, T cells
Discussion
This mini review reports on recent evidence highlighting the role of NAD+ as a key factor in the anti-tumor T cell response. To date, a handful of studies indicate that NAD+ administration may boost the anti-tumor activity of canonical and engineered T cells in preclinical models of human tumors. NAD+ depletion brought about by immunosuppressive mechanisms, which involve NAD+-consuming enzymes, may dampen T cell functions.
Based on these findings, it appears that CD38 immunosuppressive activity in human solid and hematological tumors, which hinges upon the production of the inhibitory molecule ADO, might exploit NAD+ consumption as well.
This feature is more relevant in the specific microenvironment of the BM niche in MM, which is characterized by hypoxia and low pH, thus rendering the alternative pathway (which consumes NAD+) more effective than the canonical one.
In conclusion, NAD+ balance must be taken into account in the treatment of cancer patients, and enzymes involved in NAD+ synthesis and catabolism may represent novel targets for adjuvant therapies, contributing to the success of adoptive T cell therapies.
Indeed, recent studies have addressed the efficacy of CD38 inhibitors (i.e. kuromanin) in preclinical models of CLL (33, 34) in combination with standard therapies. In addition, Nam has been administered in combination with radiotherapy or chemotherapy to patients with different solid tumors, obtaining promising effects (35).
Thus, pharmacological interventions aimed at restoring NAD+ levels may increase the efficacy of standard therapies for patients with solid and hematological tumors. In this line, other NAD+ precursors, such as NR and NMN, have been recently proposed to revert NAD+ depletion in multiple organ fibrosis (36) and aging (37), respectively. Finally, five clinical studies using Nam as adjuvant therapy are currently ongoing for cancer patients (www.clinicaltrials.gov).
https://www.mountsinai.org/health-library/supplement/vitamin-b3-niacin
People also ask
Is 60 mg of niacin too much?
High doses (50 mg or more) of niacin can cause side effects. The most common side effect is called "niacin flush," which is a burning, tingling sensation in the face and chest, and red or flushed skin. Taking an aspirin 30 minutes prior to the niacin may help reduce this symptom.